Health Innovation the CRISPR way
"Guys Google #crisper baby", a young colleague texted the
team.
 The
Googling didn’t take long to make me realize the spelling error. The word was
meant to be CRISPR (pronounced “crisper”),
alluding to the cutting edge genomic editing technology that had taken the
world by storm. The human baby (or babies, twins Lulu and Nana, to be more
precise, as I learned later) created in China using CRISPR had created a lot of
excitement, and not necessarily the good kind. The births occurred late last
year so you may think this is not newsworthy anymore. I believe it is equally
important now, given the continuing after effects; just one case in point is it
being referred to the ‘CRISPR baby scandal’ by the very prestigious journal
Nature.
The
Googling didn’t take long to make me realize the spelling error. The word was
meant to be CRISPR (pronounced “crisper”),
alluding to the cutting edge genomic editing technology that had taken the
world by storm. The human baby (or babies, twins Lulu and Nana, to be more
precise, as I learned later) created in China using CRISPR had created a lot of
excitement, and not necessarily the good kind. The births occurred late last
year so you may think this is not newsworthy anymore. I believe it is equally
important now, given the continuing after effects; just one case in point is it
being referred to the ‘CRISPR baby scandal’ by the very prestigious journal
Nature.
At
first, I didn’t realize the misspelling. In the heat of the moment (no pun
intended), I thought the term ‘crisper
baby’ was referring to some burn-related injury. The fact that I’m a
pediatric emergency physician likely informed that pseudo-deduction.
“Not only is the victim a baby, the ‘hashtag crisper
baby’ meme is even more unfortunate”, I thought.
Although
I wouldn’t put it past social media or tabloids to come up with such
cringe-worthy titles, I was intrigued.
Whenever
I’m asked by my post-millennial team to Google something, I generally comply.
Likely because of my #FOMO (and for the uninitiated in such 3-4 letter
acronyms, that stands for ‘Fear Of Missing Out’).
 The
Googling didn’t take long to make me realize the spelling error. The word was
meant to be CRISPR (pronounced “crisper”),
alluding to the cutting edge genomic editing technology that had taken the
world by storm. The human baby (or babies, twins Lulu and Nana, to be more
precise, as I learned later) created in China using CRISPR had created a lot of
excitement, and not necessarily the good kind. The births occurred late last
year so you may think this is not newsworthy anymore. I believe it is equally
important now, given the continuing after effects; just one case in point is it
being referred to the ‘CRISPR baby scandal’ by the very prestigious journal
Nature.
The
Googling didn’t take long to make me realize the spelling error. The word was
meant to be CRISPR (pronounced “crisper”),
alluding to the cutting edge genomic editing technology that had taken the
world by storm. The human baby (or babies, twins Lulu and Nana, to be more
precise, as I learned later) created in China using CRISPR had created a lot of
excitement, and not necessarily the good kind. The births occurred late last
year so you may think this is not newsworthy anymore. I believe it is equally
important now, given the continuing after effects; just one case in point is it
being referred to the ‘CRISPR baby scandal’ by the very prestigious journal
Nature.
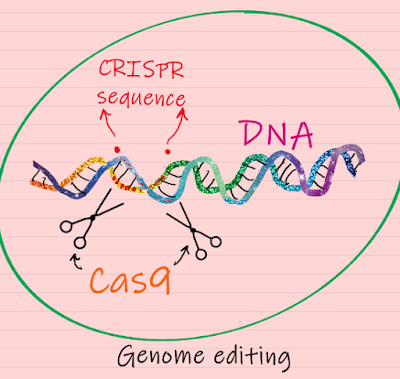
Prior
to delving into the ethical debate around it, let me explain what CRISPR is and
does. CRISPR stands for
Clustered Regularly Interspaced Short Palindromic Repeats. These are special
DNA sequences originally discovered in bacterial genomes. CRISPR works in
conjunction with a protein called Cas9 (or ‘CRISPR-associated protein 9’), an
enzyme that acts like a pair of molecular scissors that can cut strands of DNA.
Taken together, the CRISPR-Cas9 system constitutes a simple, yet powerful
editing tool for any genome. It allows researchers to easily alter DNA, which may
then lead to modification of gene function. Going into further details of how
this system works will be beyond the scope of this blog, but there are
excellent credible resources that one can access through the web, if interested
in learning further.
Gene editing has great
potential in both treating and preventing diseases that are either single gene
disorders or those that show more complex inheritance patterns. A few examples
of the former for which research is ongoing include cystic fibrosis, hemophilia
and sickle cell anemia. From the more complex disease category, there is
potential to tackle various cancers, mental illness, heart disease, and HIV
infection. The research studies tend to focus on cells and animal models. They
have not employed patients in large clinical trials as yet. But this area is
opening up. For instance, here’s one scenario: a young woman with a strong
family history of breast cancer known to carry a mutation in the BRCA1 gene
(linked with breast cancer) opts for CRISPR-Cas9-mediated gene editing through
a gene therapy clinical trial. Although this may not be science fact as yet, it
may not be too far off in the distant future.
Techniques for modifying
genomes have existed for several decades. However, recent innovations around the
technology have enabled faster, more efficient and cheaper approaches. Through
research, this field of applied biology has been prevalent in non-humans, i.e.,
‘model organisms’ such as yeast, bacteria and mice, in the pre-clinical
laboratory setting. However, the popular media only tends to take more notice
when potential usage in humans is under consideration.
As the science of genome
editing gathers momentum, so does the ethics of it. The beneficial aspect of
treating or preventing human disease using gene editing in adult somatic (or
body) cells may be self-evident. The dilemma arises when germline DNA editing
is considered. Germline refers to the reproductive cells, i.e. the sperm and
egg. Tinkering of germline DNA through gene editing means that the altered DNA
will be transmitted to future generations. The scenario of ‘genomic enhancement’ using CRISPR-Cas9 technology will not be far
behind. In other words, parents-to-be who have the money for it, will opt to
genetically enhance their babies who on top of zero likelihood of certain
genetic disorders, will have enhanced features based on mere parental whims, such as
color of skin, height, ‘beauty’ / cosmetic features, etc.
The general consensus of the
scientific and non-scientific communities alike is that we are nowhere close to
an understanding of the technology and what it can do, to give it a green
signal at this stage, either scientifically or ethically. However, what has
happened in China with the twins being products of gene-edited germline DNA, we
may have to propel this discussion ahead. We need to pause and ponder what are
we really getting into?
In the late 90s, as Y2K was
approaching, rather than being depressed about the world coming to an end, I
was really excited. I was a graduate student in human genetics and at that time
the Human Genome Project was in full swing. I was quite full of the young
physician-scientist’s chutzpah that complete knowledge of our genetic heritage and
subsequent gene therapy successes would be the panacea that the world was
seeking. Although I was lured by the love of genes and genomes, there was
something lacking towards the end of my graduate studies that precluded my
continued work in the field of genetics. I was impatient and I felt that the
speed of progress then, using genomics as a tool, was just not fast enough for
me.
Now
that I’m older and hopefully wiser, I think I tend to be more nuanced in my
approach to scientific hype. I used to get energized thinking about genomics, gene
therapy, stem cells, and such. A decade or two later, I feel the same is happening
with data science, artificial intelligence, machine learning, and the latest
craze, genomic editing. CRISPR may be a real game changer per some
proponents of the technology. Perhaps they have their own vested interests in
extolling this as the next molecular revolution. However, there is still so
much to learn and understand about CRISPR and what it can or cannot deliver,
that cautious optimism should be the order of the day. Slow and steady, prudent
progress in this field will likely go a lot further in pushing the envelope on
health innovation for the betterment of humanity the world over.
[from Health & Disease]
Acknowledgment: Author acknowledges Haider Ali and Rayaan Mian for critically reviewing and editing this essay.




I read your whole article it is really helpful for me and others. the points you described above about
ReplyDeleteeyes and its health Kjolberg is very helpful for everyone. After reading these points I am blessed to have this blog. I appreciate your work and knowledge. I will recommend this content to others, more people will get the information and help. Thanks!
Myopia is one of the most common eye problems faced by people around the world. Myopia can lead to high blindness. So regular and timely eye check up is important to better prevent and manage eye conditions. Eye Exam Singapore
ReplyDelete